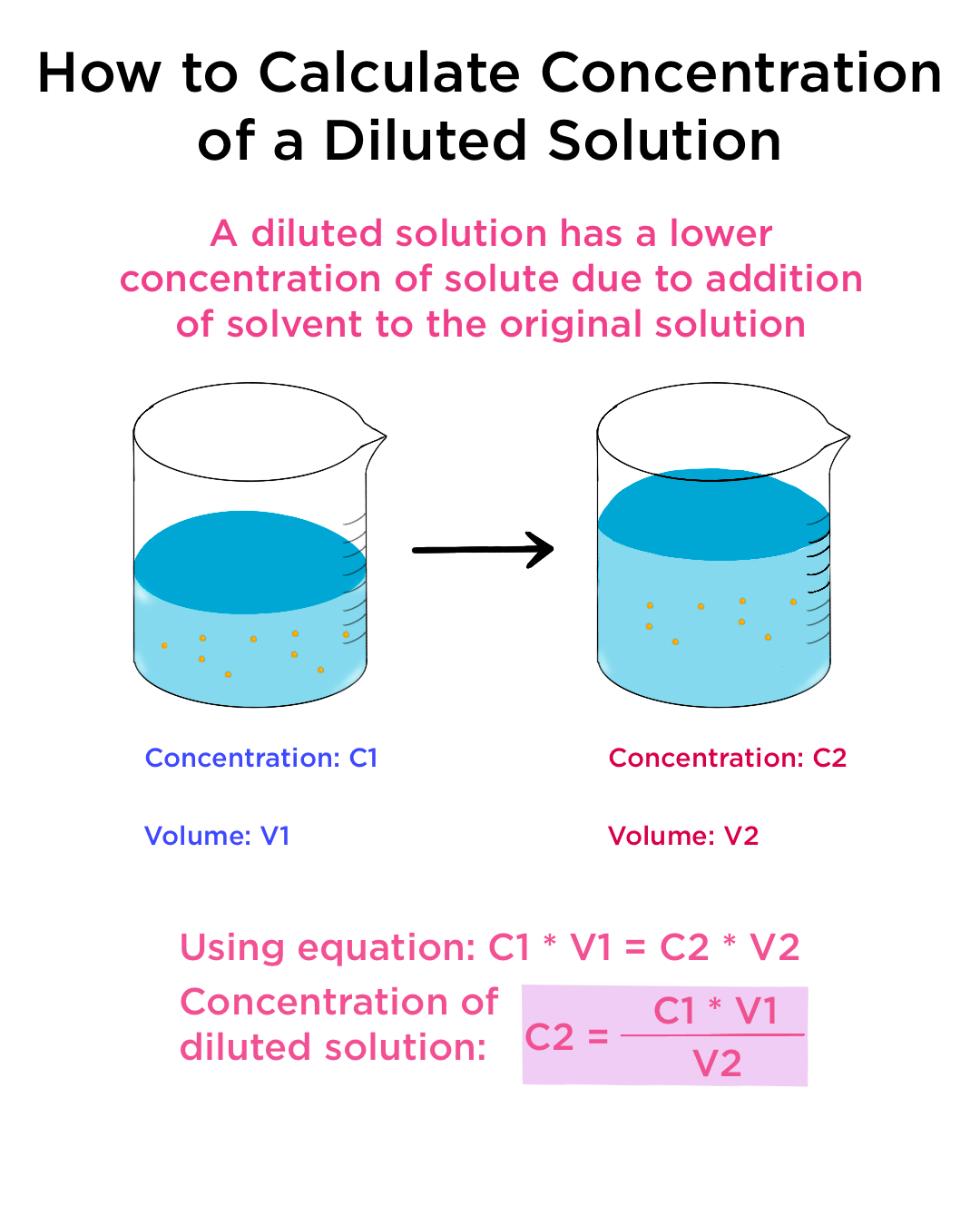
Dilution Equation With . How to make a dilution. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. We will begin our discussion of solution concentration with two related and relative terms: Follow these five steps to make a dilution: Notice that the volumes need not be converted to. Apply the dilution equation to calculate the final concentration, or the final volume, of a diluted. Usually this is done by evaporating or boiling, assuming that the heat of boiling does. Concentrating solutions involves removing solvent. If you do not know them already, use the dilution. A more simplified way of solving this is by using the dilution formula: Using the dilution equation, we write: (m1) (v1) = (m2) (v2), where m's are molarities and v's are. A dilute solution is one.
from www.expii.com
(m1) (v1) = (m2) (v2), where m's are molarities and v's are. We will begin our discussion of solution concentration with two related and relative terms: How to make a dilution. A dilute solution is one. Follow these five steps to make a dilution: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Concentrating solutions involves removing solvent. If you do not know them already, use the dilution. Apply the dilution equation to calculate the final concentration, or the final volume, of a diluted. Using the dilution equation, we write:
Dilution of Solutions — Overview & Examples Expii
Dilution Equation With A more simplified way of solving this is by using the dilution formula: Usually this is done by evaporating or boiling, assuming that the heat of boiling does. Follow these five steps to make a dilution: If you do not know them already, use the dilution. How to make a dilution. A more simplified way of solving this is by using the dilution formula: (m1) (v1) = (m2) (v2), where m's are molarities and v's are. Using the dilution equation, we write: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Concentrating solutions involves removing solvent. Notice that the volumes need not be converted to. Apply the dilution equation to calculate the final concentration, or the final volume, of a diluted. A dilute solution is one. We will begin our discussion of solution concentration with two related and relative terms:
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution Equation With Using the dilution equation, we write: A more simplified way of solving this is by using the dilution formula: Notice that the volumes need not be converted to. Follow these five steps to make a dilution: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. How to make a dilution. Concentrating solutions involves removing solvent. We will begin. Dilution Equation With.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions Dilution Equation With Concentrating solutions involves removing solvent. We will begin our discussion of solution concentration with two related and relative terms: Usually this is done by evaporating or boiling, assuming that the heat of boiling does. A more simplified way of solving this is by using the dilution formula: Using the dilution equation, we write: Notice that the volumes need not be. Dilution Equation With.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Equation With If you do not know them already, use the dilution. A dilute solution is one. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. We will begin our discussion of solution concentration with two related and relative terms: How to make a dilution. Apply the dilution equation to calculate the final concentration, or the final volume, of a. Dilution Equation With.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Equation With (m1) (v1) = (m2) (v2), where m's are molarities and v's are. If you do not know them already, use the dilution. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. A more simplified way of solving this is by using the dilution formula: Notice that the volumes need not. Dilution Equation With.
From www.slideshare.net
Molarity and dilution Dilution Equation With Apply the dilution equation to calculate the final concentration, or the final volume, of a diluted. We will begin our discussion of solution concentration with two related and relative terms: How to make a dilution. Usually this is done by evaporating or boiling, assuming that the heat of boiling does. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x =. Dilution Equation With.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID Dilution Equation With We will begin our discussion of solution concentration with two related and relative terms: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. A more simplified way of solving this is by using the dilution formula: Notice that the volumes need not be converted to. A dilute solution is one. Concentrating solutions involves removing solvent. How to make. Dilution Equation With.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Equation With A dilute solution is one. Follow these five steps to make a dilution: Concentrating solutions involves removing solvent. How to make a dilution. Notice that the volumes need not be converted to. Usually this is done by evaporating or boiling, assuming that the heat of boiling does. (m1) (v1) = (m2) (v2), where m's are molarities and v's are. We. Dilution Equation With.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Equation With A dilute solution is one. (m1) (v1) = (m2) (v2), where m's are molarities and v's are. Concentrating solutions involves removing solvent. Using the dilution equation, we write: If you do not know them already, use the dilution. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Apply the dilution equation to calculate the final concentration, or the. Dilution Equation With.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Equation With Notice that the volumes need not be converted to. Usually this is done by evaporating or boiling, assuming that the heat of boiling does. Concentrating solutions involves removing solvent. We will begin our discussion of solution concentration with two related and relative terms: Apply the dilution equation to calculate the final concentration, or the final volume, of a diluted. Using. Dilution Equation With.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilution Equation With If you do not know them already, use the dilution. Apply the dilution equation to calculate the final concentration, or the final volume, of a diluted. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. How to make a dilution. Usually this is done by evaporating or boiling, assuming that the heat of boiling does. A dilute solution. Dilution Equation With.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Dilution Equation With (m1) (v1) = (m2) (v2), where m's are molarities and v's are. A more simplified way of solving this is by using the dilution formula: Apply the dilution equation to calculate the final concentration, or the final volume, of a diluted. How to make a dilution. Usually this is done by evaporating or boiling, assuming that the heat of boiling. Dilution Equation With.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilution Equation With Using the dilution equation, we write: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Follow these five steps to make a dilution: A more simplified way of solving this is by using the dilution formula: Usually this is done by evaporating or boiling, assuming that the heat of boiling does. Concentrating solutions involves removing solvent. Notice that. Dilution Equation With.
From www.answersarena.com
[Solved] Dilution diagrams can be very helpful in organiz Dilution Equation With Concentrating solutions involves removing solvent. Using the dilution equation, we write: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. A more simplified way of solving this is by using the dilution formula: If you do not know them already, use the dilution. (m1) (v1) = (m2) (v2), where m's are molarities and v's are. Usually this is. Dilution Equation With.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Equation With Notice that the volumes need not be converted to. Using the dilution equation, we write: A dilute solution is one. If you do not know them already, use the dilution. Follow these five steps to make a dilution: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. We will begin our discussion of solution concentration with two related. Dilution Equation With.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Equation With (m1) (v1) = (m2) (v2), where m's are molarities and v's are. Using the dilution equation, we write: Concentrating solutions involves removing solvent. Usually this is done by evaporating or boiling, assuming that the heat of boiling does. If you do not know them already, use the dilution. We will begin our discussion of solution concentration with two related and. Dilution Equation With.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Equation With Concentrating solutions involves removing solvent. Using the dilution equation, we write: Follow these five steps to make a dilution: Apply the dilution equation to calculate the final concentration, or the final volume, of a diluted. (m1) (v1) = (m2) (v2), where m's are molarities and v's are. If you do not know them already, use the dilution. A dilute solution. Dilution Equation With.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Equation With Notice that the volumes need not be converted to. We will begin our discussion of solution concentration with two related and relative terms: How to make a dilution. Usually this is done by evaporating or boiling, assuming that the heat of boiling does. A dilute solution is one. (m1) (v1) = (m2) (v2), where m's are molarities and v's are.. Dilution Equation With.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilution Equation With If you do not know them already, use the dilution. A dilute solution is one. How to make a dilution. (m1) (v1) = (m2) (v2), where m's are molarities and v's are. Using the dilution equation, we write: A more simplified way of solving this is by using the dilution formula: Notice that the volumes need not be converted to.. Dilution Equation With.